After official review, the Beroni’s SARS-CoV-2 IgG/IgM Antibody Detection Kit is confirmed to comply with the quality standards and safety requirements stated in the “Announcement on Further Strengthening the Quality Supervision of Exported Anti-epidemic Materials” jointly issued by the Ministry of Commerce of the People’s Republic of China, the General Administration of Customs, and the State Administration of Market Supervision. As such, Beroni China (“Tianjin Beroni Biotechnology Co. Ltd.) is now included in the List of Medical Devices and Supplies Companies with Certification/Authorization from Other Countries by China Chamber of Commerce of Medicines & Health Products Importers & Exporters (Website: www.cccmhpie.org.cn).

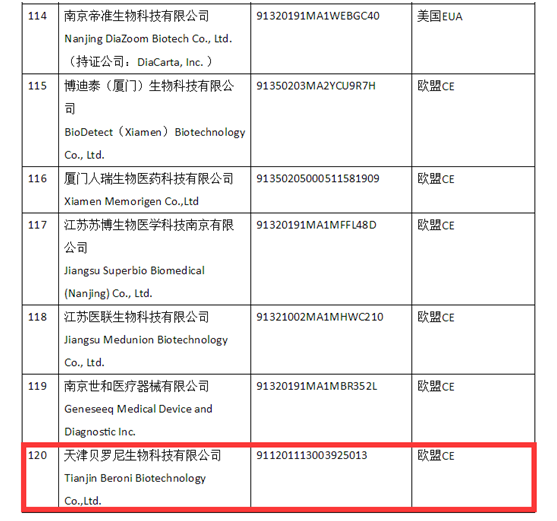
To combat the continuous spread of the COVID-19 pandemic, Beroni is committed to working with the international health community to develop new coronavirus detection kits. Beroni’s SARS-CoV-2 IgG/IgM Antibody Detection Kit has also received CE Certification in April 2020. Having completed the research and certification of the test kit, Beroni has met the formal export qualifications to sell the product to other countries.

